Referral of patients
Patients who wish to participate in the MYCHOICE study must be referred to one of the participating centers. At this moment, this includes only Isala Hospital Zwolle. Patients will be counseled at the gynecology outpatient departmentabout the MYCHOICE study. If the patient turns out to be eligible for the MR-HIFU treatment, after a screening MRI-scan is performed, she will be randomized to either the MR-HIFU treatment, or the non-MR-HIFU group. In the latter case, the patient may choose between embolization, myomectomy or hysterectomy. These treatments will take place in the hospital to which the patient has been referred, or at the refering hospital. If your patient participates in the MYCHOICE study, you will be notified. For more information or in case of questions, please contact us: mychoice@isala.nl
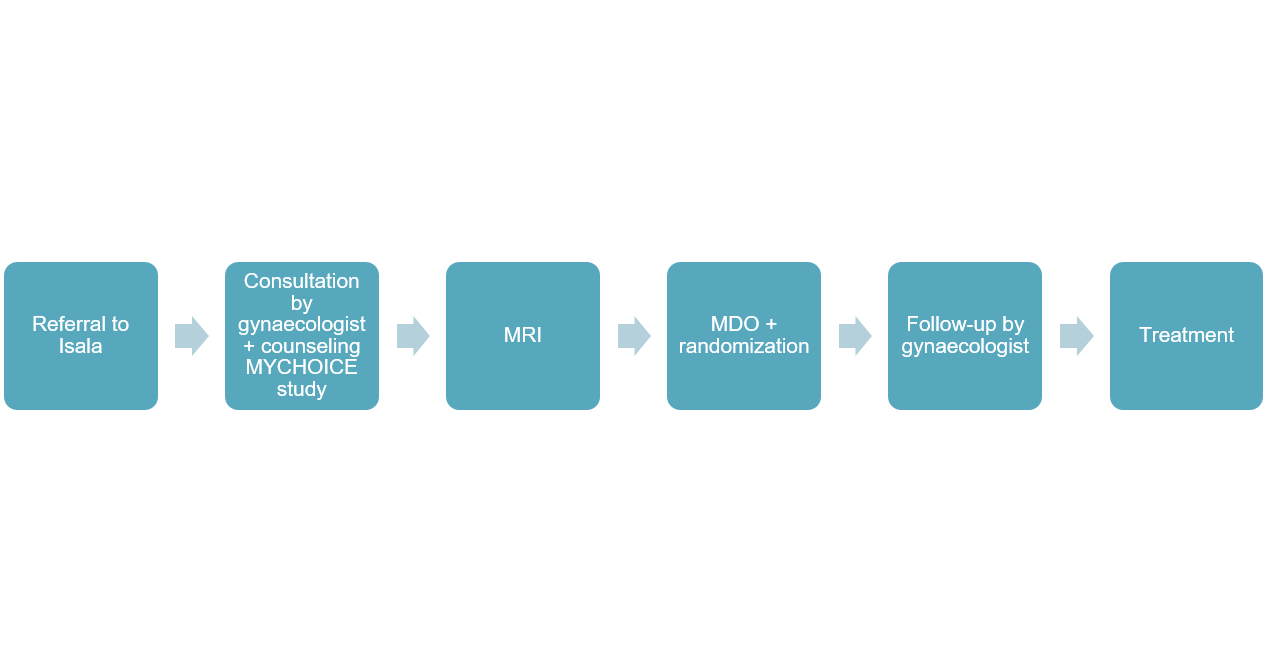
In- and exclusion criteria
Women can participate in the MYCHOICE study in case of:
- Symptomatic uterus myomatosus
- Wish for non-medication treatment (myomectomy, hysterectomy or uterine artery embolization)
- pre menopausal
- > 18 years of age
- Eligible for MR-HIFU treatment
Women can not participate in the MYCHOICE study in case of:
- BMI >35kg/m2 or abdominal subcutis >4cm
- >5 uterine fibroids, unless symptoms are caused by < 5 uterine fibroids
- MRI contraindications or contrast allergy
- Pregnancy or active pregnancy wish (<1 year)
- Suspicion of malignancy or dominant adenomyosis
- FIGO type 0-1 or 7-8
- Diameter <2cm
- Diameter >10cm, unless pre-treted with GnRH analogue
- Uterine fibroid with high signal intensity on MRI scan, unless pre-treted with GnRH analogue
- Uterine fibroid eligible for hysteroscopy
- Distance dorsal side uterine fibroid – abdominal wall >12cm
- Calcification of uterine fibroid
Screening MRI
In order to be able to decide whether a patient is eligible to undergo MR-HIFU treatment, an MRI scan will be made. This can be performed in Isala Zwolle after referral, but can also be performed in your own hospital. If necessary, these images can then be discussed in the MDO prior to referral.
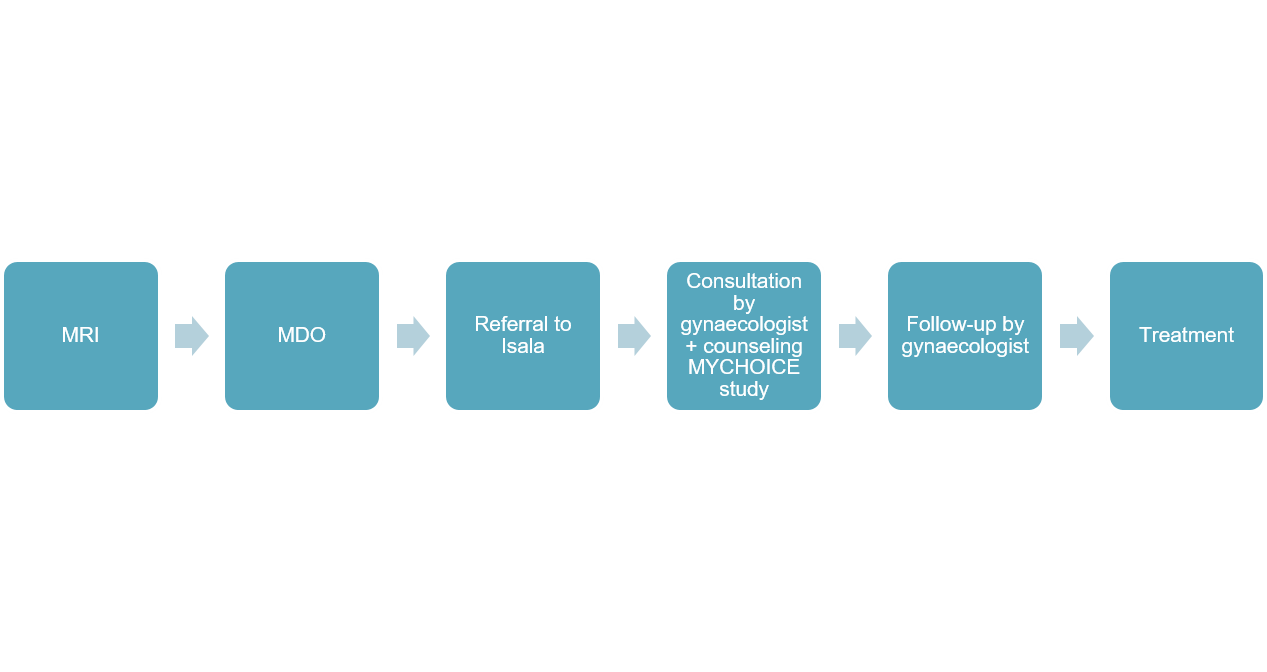
The required sequences and settings are as follows:
- T2 weighted MRI in three directions (sagittal, coronal and axial)
- T1 weighted MRI after contrast administration in minimal coronal direction
- The patient is scanned in the prone position (consistent with the position during MR-HIFU treatment)
- The complete abdominal wall of the patient is imaged
- Possibly an MRA
More details about the sequences and settings can be requested via mychoice@isala.nl
MR-HIFU treatment of symptomatic uterine myomatosus
MR-HIFU (Magnetic Resonance guided High-Intensity Focused Ultrasound) is an innovative and synergistic combination of two techniques: imaging and treatment. Using ultrasound energy, tissue can be ablated in a minimally invasive way to case necrosis (“operate without cutting”). Simultaneously, MRI imaging is used for real-time planning, control and monitoring of the result. Moreover, MRI is used in advance for the selection of patients. Currently, the most widely used application of MR-HIFU is the treatment of uterine fibroids.
Since June 2016, the treatment of fibroids using MR-HIFU has been successfully carried out in Isala Zwolle within the framework of scientific research. The University Medical Center Utrecht and Isala Zwolle are currently the only hospitals in the Benelux that offer this treatment for fibroids.
A systematic literature review and the experiences of Isala Zwolle both indicate that this new treatment method offers many advantages over common practice. The MR-HIFU treatment of fibroids is (cost-)effective, non-invasive, uterine saving, can be performed in day treatment and is associated with a short recovery time and few complications. However, the percentage of long-term re-interventions has not yet been properly investigated.
During the MR-HIFU treatment, a special transducer focuses the ultrasound energy at a certain point in the fibroid (the focal point). At this point, the tissue is locally heated above 55°C, causing protein denaturation and eventually necrosis. Several short sonications are delivered with continuous thermal feedback to measure temperature changes in the fibroid but also to check for uncontrolled heating of front and back tissues such as the “far field” including the sacral plexus or the so-called “near field” including the abdominal wall. The transducer is part of the MRI table, on which the patient lies on her abdomen during treatment. In addition to monitoring temperature changes, real-time MRI is used to determine the exact location of the treatment and to visualize the uterus and surrounding tissues.
The treated tissue of the fibroid will be (partly) absorbed by the body, causing the fibroid to shrink in the months after the treatment. It is therefore expected that the symptoms related to the fibroid will decrease. All organs and tissues around the uterus, such as the bladder, nerves and intestines will be spared. At the end of the treatment, an MRI scan with contrast will be performed so the result of the treatment can be visualized. The treatment can be performed in day treatment.
Not all women are eligible for the MR-HIFU treatment. A screening MRI-scan must show whether the fibroid can be reached sufficiently, to see there are no structures (ovaries, intestines) between the beam and the fibroid, the subcutaneous fat is not too thick and the signal intensity on the MRI scan is not too high. In addition, women must be 18 years of age or older and pre- or peri menopausal.
In the past, a pregnancy wish was a contraindication for MR HIFU treatment. However, many spontaneous pregnancies after MR HIFU have been reported. As a result, the FDA has now approved MR-HIFU treatment in women with a pregnancy wish. To date, however, there have been no randomized trials comparing pregnancy outcomes after MR HIFU treatment with other minimally invasive treatments or myomectomy. Patients who have a future pregnancy wish may participate in the MYCHOICE study if this does not involve an active pregnancy wish (<1 year).
For more information about the MR-HIFU treatment, you can contact us via the contact form.
Frequently asked questions by patients
Since 2016, MR-HIFU treatment of fibroids has been applied in Isala Zwolle. Below we provide a brief overview of frequently asked questions.
1 How likely is it that this treatment will improve my symptoms?
Answer: A systematic review of our own group showed that after twelve months, symptoms decreased by an average of 60%. Our experience is that mechanical symptoms decrease within three months, bleeding symptoms often improve between three and six months after MR-HIFU treatment.
2. How fast does the fibroid shrink?
Answer: Our own data showed that six months after a technically successful treatment, a fibroid has shrunk by an average of 45%. Literature shows that the fibroids can shrink even further thereafter. The fibroid is never expected to go away completely.
3. How great is the chance that I will still have to undergo another treatment?
Answer: The literature showed reintervention rates between 0-21%. Our experience is that 15% of the women still undergo another treatment. This means that 6 out of 7 women are sufficiently satisfied after MR-HIFU.
4. How long is the recovery after MR-HIFU? Are there any rules of conduct?
Answer: On average, women recover after three days. We do not give any behavioral rules or additional medication after discharge. The treatment takes place in day treatment.
5. What are the disadvantages of MR-HIFU?
Answer: The disadvantages of MR-HIFU are threefold: firstly, there is the chance that the patient may not be suitable for MR-HIFU. At present, 50-75% of the women referred to us are suitable, or may be suitable after pre-treatment with a GnRH analogue. Even if a patient is considered suitable, the treatment may not be technically successful due to insufficient tissue heating or failure to reach the tissue. Finally, the treatment may be technically successful, but not improve symptoms sufficiently or new symptoms appear over time. Then an alternative treatment may be chosen.
6. What are the advantages of MR-HIFU?
Answer: The treatment takes place in day care, under sedation and is non-invasive. Recovery is several days and the complication risk low. The uterus and ovaries are preserved and the treatment gives improvement in symptoms in most of the women.
7. What are the risks of MR-HIFU?
Answer: Although non-invasive, MR-HIFU does come with some risks. In addition to pain (like menstrual cramps), nausea and muscle pain on the treatment day itself, heavy menstruation may occur in the days following treatment. Very rare complications include skin burns, damage to the bladder, intestines or ovaries, stimulation of bone nerves, a urinary tract infection due to the bladder catheter or the development of a fever.
Summary of study protocol
Rationale: Roughly 1.8 million Dutch women have uterine fibroids, with 25% reporting complaints. Symptomatic fibroids have a significant impact on Quality of Life (QoL). Conservative treatment fails in 50% of patients, resulting in an indication for an invasive procedure. A hysterectomy is the most common invasive treatment, but has a high risk of complications and long recovery times. Myomectomy is uterus saving, but comes with long recovery and high risk of complications as well. This leads to a strong desire for less invasive treatment options, especially in women who want to conceive. Currently, uterine artery embolization (UAE) is the only reimbursed minimally invasive treatment option in the Netherlands. Magnetic Resonance image guided High Intensity Focused Ultrasound (MR-HIFU) is a rather new non-invasive treatment option. It is safe, (cost)effective and has a very short recovery time and fewer complications compared to surgical intervention and UAE. However, a lack of comparative information on long-term (cost)effectiveness of the MR-HIFU treatment compared to the standard of care, keeps the MR-HIFU treatment from being reimbursed.
Objective: To determine the long-term (cost)effectiveness of Magnetic Resonance image guided High Intensity Focused Ultrasound (MR-HIFU) compared to standard (minimally) invasive fibroid care including UAE, myomectomy and hysterectomy.
Study design: The MYCHOICE study is a national, multicenter, open randomized controlled trial with randomization in a 2:1 ratio to MR-HIFU or standard care including hysterectomy, myomectomy and UAE. The non-inferiority margin is set to 15 points SSS reduction of the UFS-QoL (range: 0-100). With an estimated drop-out of 20%, the sample size is 240 patients in 2:1 ratio for MR-HIFU (n=160) and standard care (n=80). Both an intention-to-treat and per protocol analysis will be performed. Follow-up will be 24 months.
Study population: In brief: Women ≥18 years, premenopausal, diagnosed with symptomatic uterine fibroids in whom conservative treatment failed or is undesired, eligible for MR-HIFU.
Exclusion criteria: MRI contra-indications, suspicion of malignancy, dominant adenomyosis, BMI ≥35kg/m2, currently pregnant or active wish to conceive and not able or willing to sign informed consent.
Intervention: The studied intervention MR-HIFU, leads to significant symptom reduction, less complications, the possibility to conceive and faster recovery than current standards of care.
Main study endpoints: Primary outcomes of the study are the QoL at 24 months after treatment (measured by the UFS-QoL symptom severity score (SSS)) and the costs consisting of direct health care costs, loss of productivity and patient costs. Secondary outcomes include time-interval between treatment and re-intervention, adverse events (AE’s)/complications, patient reported outcome measures (PROM’s), patient reported experience measures (PREM’s), pregnancy outcomes, onset of menopause, length of hospital stay, peri- and post procedural pain, (co)medication use and non-perfused volume/fibroid shrinkage.
Nature and extent of the burden and risks associated with participation, benefit and group relatedness: By collecting data on the long-term (cost)effectiveness of the MR-HIFU treatment in comparison to the current standard of fibroid care, we provide currently unavailable evidence about the proper place of MR-HIFU in the fibroid treatment spectrum. We expect our study to lead to reimbursement and implementation of the intervention in national uterine fibroid care guidelines, making the treatment available for all uterine fibroid patients. Based on the current evidence, we believe that the risk for the patient undergoing the intervention is very small, especially when compared to the expected individual gains: uterus preservation and short(er) recovery times leading to less productivity loss. In terms of burden, all patients need to fill in a questionnaire before and four times after treatment.
Research protocol: Anneveldt KJ, Nijholt IM, Schutte JM, et al., MYoma treatment Comparison study: High intensity image guided fOcused ultrasound versus standard (minimally) Invasive fibroid care – a (Cost) Effectiveness analysis (MYCHOICE): Study protocol for a multicenter randomized controlled trial. JMIR Res Protoc 2021;10(11):e29467 doi:10.2196/29467
About us
The MYCHOICE study was designed and set up by the MR-HIFU team of Isala Zwolle. This team consists of radiologist and principal investigator dr. M.F. Boomsma, gynecologist dr. J.M. Schutte, gynecologist drs. J.R. Dijkstra, senior scientist and epidemiologist dr. I.M. Nijholt and physician-researcher drs. K.J. Anneveldt.
Outside Isala, we are supported by the local investigators. Gynecologist Prof. Dr. A. Franx, HTA expert Dr. G.W.J. Frederix, implementation expert Dr. E. Ista, the patient organizations Ikone and Bekkenbodem4all and the professional societies NVOG and NVvR support the team.
Publications by our research group
To date, the MR-HIFU research group has several scientific publications in peer-reviewed journals.
- Anneveldt KJ, Nijholt IM, Schutte JM, et al., MYoma treatment Comparison study: High intensity image guided fOcused ultrasound versus standard (minimally) Invasive fibroid care – a (Cost) Effectiveness analysis (MYCHOICE): Study protocol for a multicenter randomized controlled trial. JMIR Res Protoc 2021;10(11):e29467 doi:10.2196/29467
- Verpalen IM, Anneveldt KJ, Nijholt IM, et al. Magnetic resonance-high intensity focused ultrasound (MR-HIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: A systematic review and meta-analysis, Eur J Radiol 2019;120:108700 doi:S0720-048X(19)30350-X
- Verpalen IM, de Boer JP, Linstra M, Pol RLI, Nijholt IM, Moonen CTW, Bartels LW, Franx A, Boomsma MF, Braat MNG. The Focused Ultrasound Myoma Outcome Study (FUMOS); a retrospective cohort study on long-term outcomes of MR-HIFU therapy. Eur Radiol. 2020 May;30(5):2473-2482. doi: 10.1007/s00330-019-06641-7.
- Verpalen IM, Anneveldt KJ, Vos PC, et al. Use of multiparametric MRI to characterize uterine fibroid tissue types, MAGMA 2020 doi:10.1007/s10334-020-00841-9
- Anneveldt KJ, van ‘t Oever HJ, Nijholt IM et al. Systematic review of reproductive outcomes after High Intensity Focused Ultrasound treatment of uterine fibroids, European Journal of Radiology 2020 doi: 10.1016/j.ejrad.2021.109801
- Verpalen IM, van ‘t Veer-Ten Kate, M., de Boer E, et al., Development and clinical evaluation of a 3-step modified manipulation protocol for MRI-guided high-intensity focused ultrasound of uterine fibroids. Eur Radiol 2020. doi: 10.1007/s00330-020-06780-2
- Verpalen IM, de Boer JP, Linstra M, et al., The Focused Ultrasound Myoma Outcome Study (FUMOS); a retrospective cohort study on long-term outcomes of MR-HIFU therapy. Eur Radiol 2020;30(5):2473-82. doi: 10.1007/s00330-019-06641-7
- Anneveldt KJ, Verpalen IM, Nijholt IM, Dijkstra JR, van den Hoed RD, Van’t Veer-Ten Kate M, de Boer E, van Osch JAC, Heijman E, Naber HR, Ista E, Franx A, Veersema S, Huirne JAF, Schutte JM, Boomsma MF. Lessons learned during implementation of MR-guided High-Intensity Focused Ultrasound treatment of uterine fibroids. Insights Imaging. 2021 Dec 18;12(1):188. doi: 10.1186/s13244-021-01128-w.
- Anneveldt KJ, van ‘t Oever HJ, Verpalen IM, Nijholt IM, Bartels W, Dijkstra JR, van den Hoed RD, van ‘t Veer-Ten Kate M, de Boer E, Veersema S, Huirne JAF, Schutte JM, Boomsma MF. Increased MR-guided high intensity focused ultrasound (MR-HIFU) sonication efficiency of uterine fibroids after carbetocin administration. Eur J Radiol Open. 2022 Mar 21;9:100413. doi: 10.1016/j.ejro.2022.100413.
- Slotman DJ, Bartels LW, Zijlstra A, Verpalen IM, van Osch JAC, Nijholt IM, Heijman E, van ‘t Veer-Ten Kate M, de Boer E, van den Hoed RD, Froeling M, Boomsma MF. Diffusion-weighted MRI with deep learning for visualizing treatment results of MR-guided HIFU ablation of uterine fibroids. Eur Radiol. 2023 Jun;33(6):4178-4188. doi: 10.1007/s00330-022-09294-1.
- Slotman DJ, Nijholt IM, Schutte JM, Boomsma MF. No incision required for long-lasting symptom relief in a selection of women suffering from uterine fibroids. Eur Radiol. 2023 Nov;33(11):7357-7359. doi: 10.1007/s00330-023-10197-y.
- Anneveldt KJ, Nijholt IM, Schutte JM, Hehenkamp WJK, Veersema S, Huirne JAF, Boomsma MF. Waste analysis and energy use estimation during MR-HIFU treatment: first steps towards calculating total environmental impact. Insights Imaging. 2024 Mar 22;15(1):83. doi: 10.1186/s13244-024-01655-2.
- Knorren ER, de Ridder LA, Nijholt IM, Dijkstra JR, Braat MNGJA, Huirne JAF, Boomsma MF, Schutte JM. Effectiveness and complication rates of high intensity focused ultrasound treatment for abdominal wall endometriosis: A systematic review. Eur J Obstet Gynecol Reprod Biol. 2024 Jun;297:15-23. doi: 10.1016/j.ejogrb.2024.03.029.
Looking for a new collaboration?
In case you are interested in collaborating with the MYCHOICE research team, please contact us via the contact form or via mychoice@isala.nl.














